About service
C&R Research provides a full range of clinical data management services including CRF (Case Report Form) design development, database lock and regularity submission data set transformation. Across a broad range of therapeutic areas, we have extensive data management experience for Phase I to III clinical trials, clinical trials for approval of a new medical device and post-approval Phase IV clinical trials, observational study (OS)/non-interventional study (NIS), investigator-initiated trial (IIT), and post-marketing surveillance (PMS) study. C&R Research offers specialized data management services that can ensure the collection and management of high-quality clinical data. In addition, to ensure the integrity of clinical data, electronic data capture (EDC) system and its system management is fully validated via effective collaboration with C&R Research’s Clinical Operation team, and data cleaning through query management process is rapidly available during the length of a clinical trial. Of the collected clinical data, data validation is periodically performed using a statistical analysis system (SAS) and all records are retained via audit trail, thus making data management transparent. This allows a regulatory authority to accelerate their speedy data review in the approval process.
Data Management
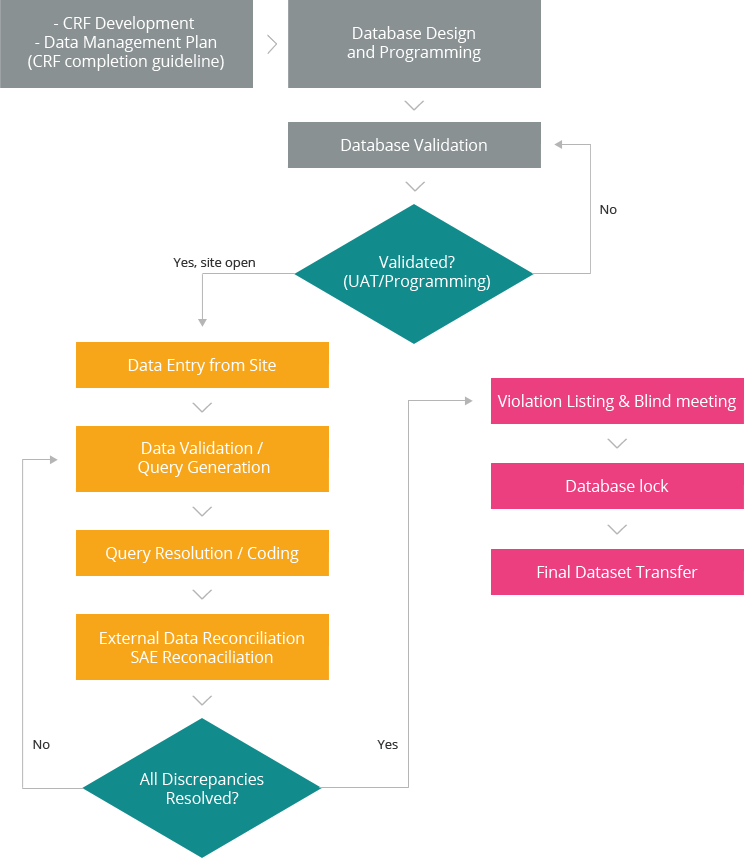
- CRF (Case Report Form) Design Development
- DMP (Data Management Plan) Development
- DB Spec (Database Specification) Development
- DVS (Data Validation Specification) Development
- CRF Completion Guideline Development
- EDC (Electronic Data Capture) System Validation & Management
- Paper Based CRF Data Management Process including Double Independent Data Entry & Quality Control)
- Database Design & Validation
- Data Validation
- Lab Data Handling
- External Data Handling
- Query Management
- Medical Coding : MedDRA, K-MedDRA, WHOART, WHODrug
- Data Quality Control
- Violation List Generation
- Database Lock
- Data Transfer
- SDTM (Study Data Tabulation Model)
- Data Back-Up & Archiving
- Independent Evaluation Data Management Process
- PMS (Post-Marketing Study) / OS (Observational Study) / NIS (Non-Intervention Study) Data Management Process
Data management services tailored for clients
C&R Research builds a broad range of data management service portfolio for all clinical trials (e.g., Phase I to III clinical trials, clinical trials for approval of a new medical device and post-approval Phase IV clinical trials, OS/NIS, IIT, and PMS) and delivers services in several EDC environments including LeadTrial EDC, Medidata Rave, and CRS Cube. Thus, C&R Research provides data management services tailored to the needs of clients by suggesting each EDC system customized to their project and budget and establishing standard interfaces that are optimized to clients’ requirements.
Global Standard Requirement Satisfied.
To provide better quality data management services in situations where domestic pharmaceutical and biopharmaceutical companies are turning their eyes on the overseas markets, C&R Research has adopted the Study Data Tabulation Model (SDTM) as the standard specification for submitting tabulation data to a regulatory authority, thus improving data quality to the global level.
Reliability assurance of data
C&R Research’s Data Management team has vast experience across a broad range of therapeutic areas, and our experts holds advanced certificates (SAS Advanced certification, MedDRA Coder certification, SDTM certification). They provide the best data management services and assures the reliability of data delivered to clients.
For more information