About service
C&R Research has expanded its regulatory affairs services to include all phases of Medical Devices development, ranging from early pre-IND Development stage to Approval IND and Manufacturing by Ministry of Food and Drug Safety (MFDS). We carefully suggest all of efficient strategies and alternatives during each step of the regulatory process towards successful product approval. Our regulatory affairs team is composed of professionals who have extensive experience and expertise in various clinical fields and help clients register product without costly delays.
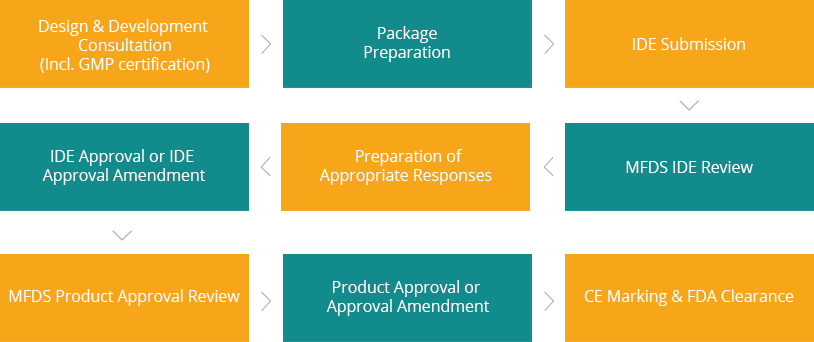
Design & Development Consultation
Package Preparation & IDE Submission to MFDS
Follow Up after MFDS Submission
Product Approval, CE Marking & FDA Clearance
Guidance on strategic direction
C&R Research’s professionals offer high-level regulatory strategies to expedite the Medical Device development process from pre-IND stage to MFDS’s IND approval and Manufacturing Permition , as part of efforts to expedite products through clinical trials and into the market. When necessary, our consulting services are provided for MFDS meeting and manufacturing Development or Medical Writing (protocol, etc.) They are always flexible and responsive to MFDS’s questions and provide strategic opinions that may eventually lead to MFDS’s IND approval and Manufacturing Permition upto Overseas certification.
Vast experience and expertise in regulatory affairs service
C&R Research employs a great number of experts with master’s and doctorate degrees. Their extensive experience in regulatory affairs at home and abroad helps clients prepare registration dossier for MFDS’s approval. Examples of these experiences, We have clinical experience and licensing experience for domestically developed high-tech medical devices including vascular stents, anti-adhesion agents, biomaterials for breakfast restoration (Filler) and AI software.
Further, C&R Research’s experts have extensive backgrounds across a broad range of therapeutic areas including Oncology, so they can suggest more specialized opinions. Reliable consulting services are also available through medical advisors
For more information