We provide customized, end-to-end post-approval research services
leveraging real-world evidence (RWE) and real-world data (RWD),
from study design that reflects actual clinical practice to
data collection and analysis, as well as regulatory responses and reporting,
to evaluate treatment effectiveness and safety in real-world healthcare settings.

(RWE) Study Design
We contribute to demonstrating post-approval value through study designs based on real-world clinical practice, enabling the evaluation of a drug’s long-term safety, compliance, and usage patterns. Our expertise covers a wide range of RWE study types, including observational studies, pharmacovigilance, registry studies, and retrospective studies, and we recommend the most suitable study type aligned with each project’s objectives. We have a deep understanding of the unique data structures and analytical methodologies of RWE research, which differ from traditional clinical trials, and utilize this expertise to deliver research outcomes with a balanced integration of quantitative and qualitative evidence.
Capability in Medical Institutions
Through close collaboration with major hospitals and medical institutions in Korea, we enhance the efficiency of subject recruitment and data collection, while strengthening the predictability of trial schedules. By leveraging real-world data such as EMR (Electronic Medical Records), health insurance claims data, and pharmacy prescription data in both study design and execution, we ensure the practicality of our research. Taking into account the differences between research environments and actual clinical settings, we implement participant engagement strategies and IRB/institutional response plans to ensure the smooth operation of RWE projects.
High-Quality Study Reports
In collaboration with data science and biostatistics experts, we establish analysis frameworks tailored to real-world data (RWD) and provide highly reliable interpretations. To ensure outputs that can be used for both academic and regulatory purposes, we deliver structured reports aligned with project objectives. With the participation of medical writing specialists, we present complex data in a clear and scientifically accurate manner, supporting the creation of high-quality documents that can be presented and submitted.
Experience
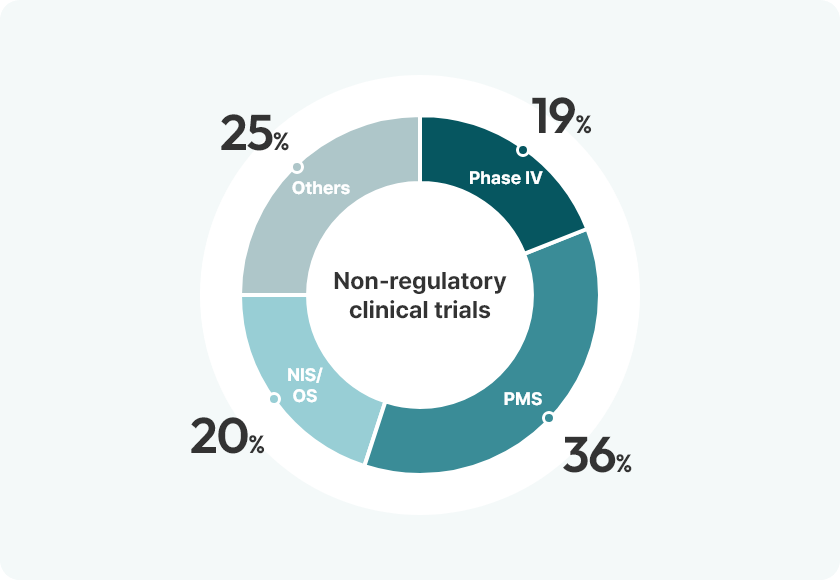
[2010-2025.1Q, Total: 629 Studies]
clinical development with C&R Research.
