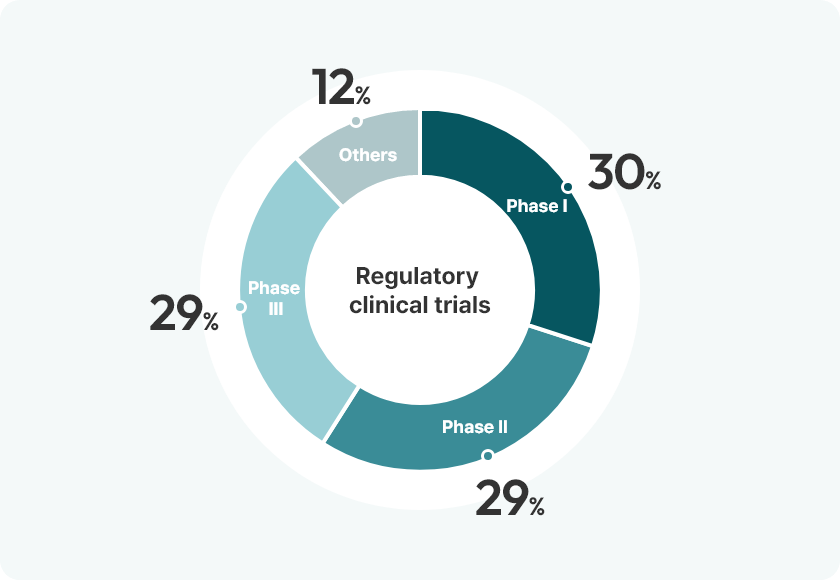
Leveraging expertise across all phases from Phase 1 to Phase 3, we provide fully
customized support throughout the entire clinical trial process, from study planning
and execution to data management and final report preparation,
tailored to meet each client’s specific needs.

Trial Design
We develop tailored clinical trial strategies based on the specific characteristics of the therapeutic area, its indication, and stage of development, ensuring efficient project management from initial planning through execution. Our integrated approach aligns each component, such as protocol design, trial site selection, and patient recruitment planning, with the overall development strategy. Through a flexible operational framework, we enable the rapid and reliable conduct of trials while providing predictable timeline management and effective risk-mitigation strategies.
Expertise and Medical Network
Drawing on extensive project experience across diverse therapeutic areas, including oncology, immunology, central nervous system (CNS) disorders, and rare diseases, we develop indication-specific strategies tailored to each therapeutic areas. By collaborating with leading domestic and international investigators and Key Opinion Leaders (KOLs) active in these areas, we enable more effective patient recruitment and ensure access to high-quality medical feedback. Through optimized trial designs and participant engagement strategies customized for each indication, we deliver differentiated clinical trial solutions.
a Global Standard Quality System
We ensure standardized and reproducible clinical trial operations based on an SOP framework that complies with international guidelines such as ICH-GCP, the Ministry of Food and Drug Safety (MFDS), the U.S. Food and Drug Administration (FDA), and the European Medicines Agency (EMA). Through professional monitoring, we conduct continuous quality checks across all areas, including trial site management, document review, and reporting systems, applying a risk-based approach. All clinical documents and data are managed using electronic systems and quality management tools, with robust systems in place to ensure data integrity and audit traceability.
Experience
[2010-2025.1Q, Total: 932 Studies]
clinical development with C&R Research.
