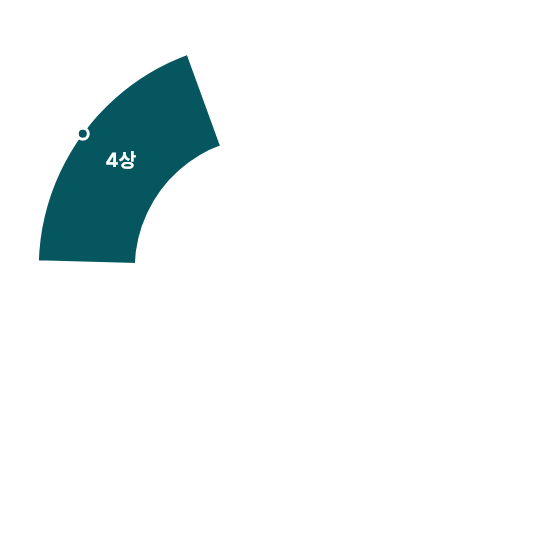
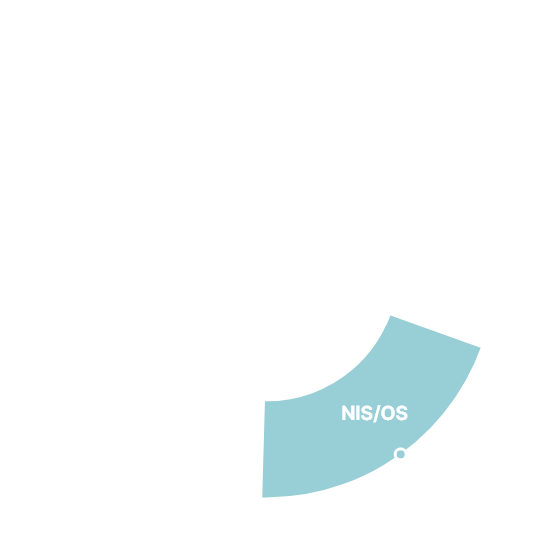
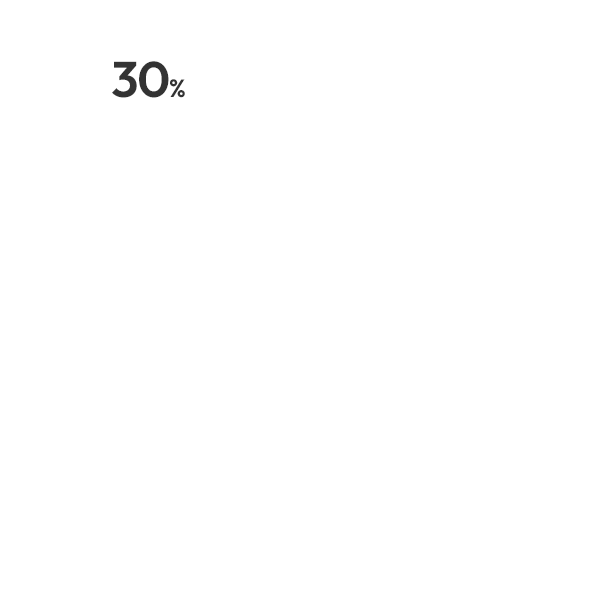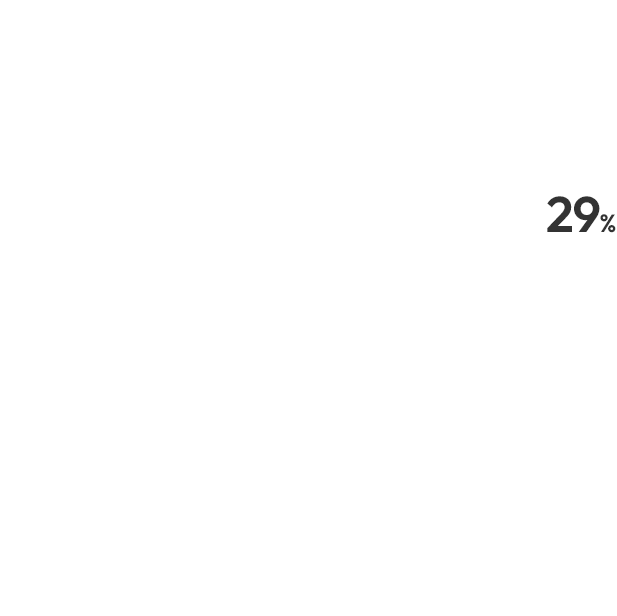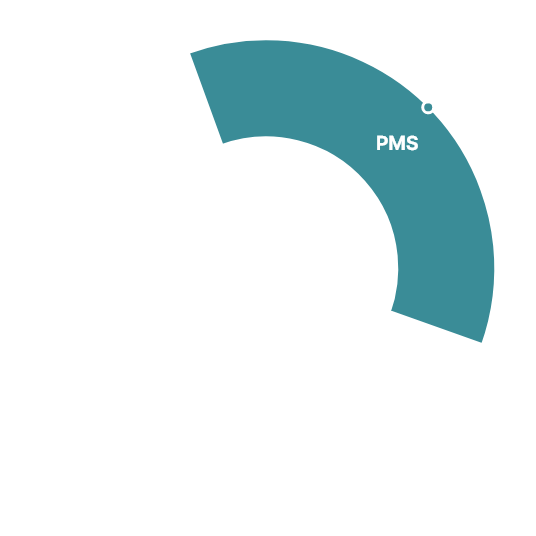Navigator of Clinical Trials
C&R
Navigator of
Clinical Trials
C&R
Navigator of Clinical Trials
C&R
Navigator of
Clinical Trials
C&R

C&R Research Drives Success
In Clinical Pathways
In Clinical Pathways
씨엔알리서치는 임상시험의
새로운 길을 개척합니다
새로운 길을 개척합니다



End to End
Service Provider
C&R
-
Since 업력 28
-
Member 직원수 500
-
Clinical Trials 임상시험수 1,800
-
Global 다국가 임상시험수 59
Full Scope Service
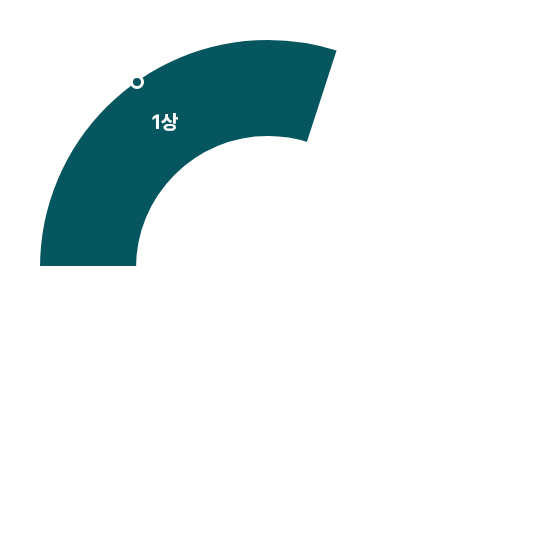
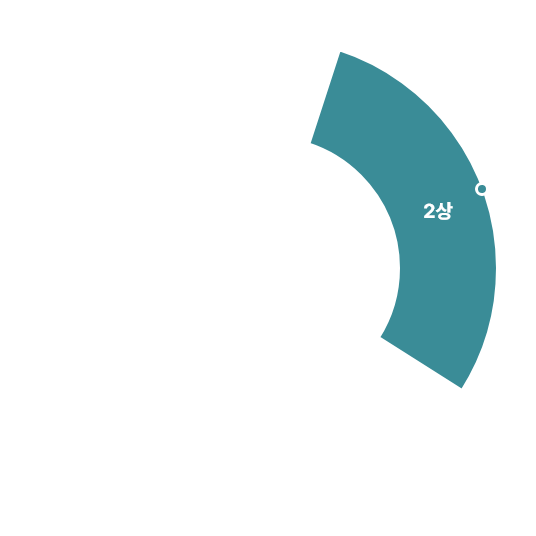
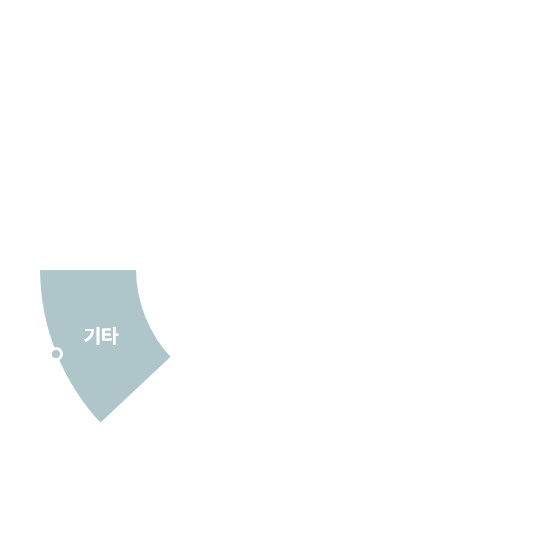
허가용 임상
비허가용 임상
[2010-2025.1Q]
Prominent Network

Leading the
Future of Clinical Trials
With Integrated Services
Future of Clinical Trials
With Integrated Services
News

Be The Best,
Lead First